To answer that question, first, try to remember if you have ever been to the Moon before.
If you have been to the Moon, then chances are you were probably dressed like this. There are many reasons you should wear a spacesuit on the Moon. It will make sure you have oxygen to breathe. It will protect you from getting knocked unconscious by minuscule meteorites (for realsies). And it will keep you from being roasted and/or frozen to death.
Unlike the Earth, it is very easy to be roasted and also frozen on the Moon, sometimes within seconds of each other. If you stood on the Moon in the sunlight while wearing shorts and a t-shirt, your temperature would quickly rise to 250 degrees Fahrenheit (121 degrees Celsius). That is more than hot enough to boil water. But walk over a few feet into the shade of a big rock and your temperature would drop to 120 degrees below zero Fahrenheit (-84 degrees Celsius). That's a 370 degree Fahrenheit difference that happens by taking a few steps. Wearing a spacesuit would reflect the heat away during the times you’re in the sun and insulate you from the cold when you’re in the shadows so you could walk around on the Moon without quickly becoming dead.
The Earth and the Moon are both pretty much the same distance from the Sun...
...but there is nowhere on the surface of Earth where you will naturally experience 250 degrees Fahrenheit temperatures (well, except if you jump into a hot spring or an active volcano). And you would have to go to Antarctica on an abnormally cold day to experience 120 degrees below zero Fahrenheit.
So, what gives? Isn’t the Sun providing the Earth with the same amount of heat as it does the Moon? Why aren’t Earthlings alternating between being broiled and frozen to death every time we walk in and out of a gazebo?
The Sun is providing the Earth and the Moon with pretty much the same amount of heat. The reason the Earth does not have ridiculously extreme temperature changes like the Moon does is because the Earth has its own built-in spacesuit. This spacesuit, which not only protects us from extreme temperatures, but also provides us with oxygen and protects us from being hit by minuscule meteorites, is called the atmosphere.
The atmosphere is the stuff around us we call the air. Even though you can’t see the air, smell the air, or taste the air (if you can see, smell, and taste the air, you may want to move to another town), the atmosphere is filled with gazillions of different molecules, which are little things so tiny you can’t see them even with a microscope. You can feel air molecules, though. If you wave your hand toward your head, the breeze you feel is a bunch of molecules smacking you in the face.
The molecules in Earth’s atmosphere are things such as nitrogen, oxygen, and carbon dioxide that are being gassy (a more accurate way to say that is: they are in their "gaseous state").
Some of these gaseous molecules, called “greenhouse gases,” are what keep the Earth’s atmosphere warm and its temperatures pretty much stable. Greenhouse gases do not warm the atmosphere by making heat, though. Instead, they trap heat from the sun.
The sun is sending heat to the Earth through its rays. Most of the sun's rays that pass through the atmosphere are in the form of ultraviolet waves (the radiation that sunblock protects you from) and visible light (the radiation that allows you to see). Ultraviolet and visible light waves can travel right through the atmosphere (though about half of these waves get blocked by clouds and other things in the atmosphere, which is a good thing for us). When sunlight waves reach the ground, the surfaces absorb their heat. As the ground warms up, that heat eventually will start rising back in the air (when you see a hawk or a vulture soaring around in a circle without flapping its wings, it is able to do so because it is riding rising hot air that was heated by the sun-baked ground). This heat is not released as ultraviolet light (you won’t get a sunburn from the ground) or visible light (you don’t need sunglasses to look at the ground). Instead, because some of the energy of the waves is lost during absorption, the heat from the ground is released as infrared radiation.
Here is a diagram of the part of the electromagnetic spectrum that we experience most directly because of sunlight. The farther you look to the right of this diagram, the more energy the waves have. So ultraviolet waves have a lot more energy than infrared waves. And if you are wearing a purple shirt (i.e. a violet shirt), it is reflecting higher energy visible light waves than when you wear a red shirt.
Though greenhouse gases cannot stop ultraviolet or visible light radiation, they can stop infrared radiation heat, at least for a little while. In doing so, greenhouse gases act kind of like a blanket. If you have ever gone to your Great-Grandmother’s house in the winter and had to sleep in the room that she doesn’t bother to heat because normally no one sleeps in there, then you know as you first tuck into that freezing cold bed that blankets are not naturally warm. Eventually, they warm up, though, thanks to the heat in your body. The inside of your body is about 98.6 degrees Fahrenheit and that heat continuously leaves your body and warms the air trapped under the blanket. Eventually, that makes you feels nice and toasty under the blanket, and just your face freezes all night.
Greenhouse gases are like a blanket around the Earth. They trap heat in our atmosphere to keep us warm. * (That asterisk indicates there is a footnote at the bottom of this post you may want to read.)
We have known about the powers of greenhouse gases for over 150 years. Back in the mid-Nineteenth Century, scientists like Eunice Foote did experiments to see how different kinds of gases affect temperature. For her most famous experiment, Eunice put thermometers into bottles. She then used an air pump to remove all the air from the bottles . One bottle was left without any air in it. Another bottle was filled with just carbon dioxide, another one with just oxygen, and the fourth bottle with the regular air you are breathing right now. The bottles were tightly sealed and then left out in the sun all day. She kept checking the temperature inside each bottle. She observed again and again that the bottle with just carbon dioxide in it became much warmer than the other bottles filled with oxygen, regular air, or nothing, indicating that carbon dioxide was much better at holding onto heat than the other gases. (You can read the original description of her experiment here.)
Since Eunice published these findings, many other scientists have done experiments that confirm that carbon dioxide stays warm much longer than most other gases. These experiments helped scientists realize that the carbon dioxide is a greenhouse gas and one of the main reasons our atmosphere is as consistently warm as it is.
Not all gases are greenhouse gases, though. Oxygen and nitrogen, which are way more abundant in our atmosphere than carbon dioxide, are so bad at trapping heat that, if they were on show called "America’s Got Heat-Trapping Talent," they would be buzzed off the stage almost immediately.
Carbon dioxide, and other greenhouse gases, are better at holding heat than oxygen and nitrogen because carbon dioxide gas molecules have more parts than oxygen and nitrogen gas molecules do.
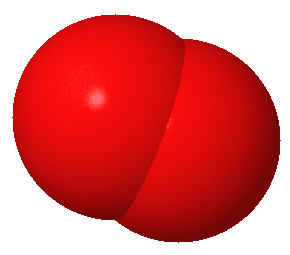
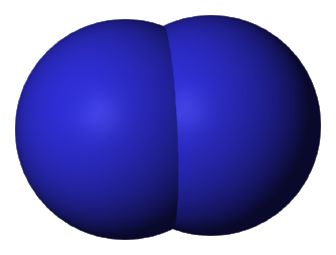
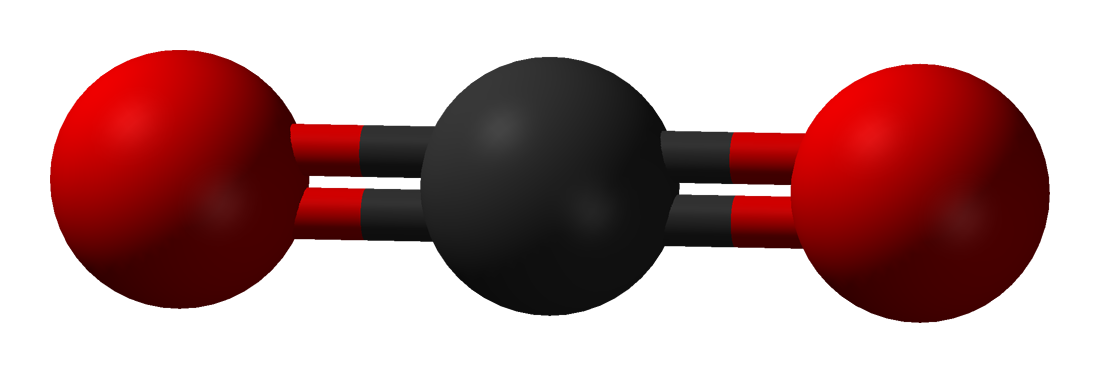
Here are simplified and creatively colored illustrations of what these three gas molecules look like.
The carbon dioxide molecule in this illustration has parts sticking out that almost look like arms (or like an Imperial TIE fighter). The oxygen and nitrogen molecules do not have parts that look like arms (they are actually in the unfortunate situation, at least in these illustrations, of looking kind of like a butt). The more parts a molecule has, the better it will be at trapping heat energy.
Heat is basically energy. The warmer something is, the more energy it has. When energy meets gas molecules, the energy makes the gas molecules vibrate, kind of like they are dancing. The gas molecules hold the energy as long as they keep vibrating.
Nitrogen and oxygen gas are simple molecules without many parts. When heat energy hits them, the best they can do is sway a tiny bit, kind of like a Weeble.
Carbon dioxide gas molecules are complex with more parts to them, so when heat energy hits them, they can vibrate around with crazy dance moves, kind of like Elaine Benes.
Because their extra parts allow them to do more dance moves than oxygen or nitrogen molecules can do, carbon dioxide and other greenhouse gas molecules hold the heat energy in the atmosphere longer than non-greenhouse gas molecules can. A molecule cannot hold the energy forever, though. When the heat leaves a carbon dioxide molecule, it is usually trapped by another greenhouse gas molecule, which holds it a little while and then releases it to be caught by another greenhouse gas molecule. It’s kind of like a game of atmospheric Hot Potato, except the “hot potato” in this game is invisible, actually hot, and there are gazillions of these hot potatoes of heat energy being tossed around.
As the greenhouse gas molecules continuously play Hot Potato with heat energy, some of the heat escapes into space. That doesn't make us freeze because the lost heat is replaced everyday by the Sun.
Greenhouse gases keep passing the Sun's heat energy around the atmosphere, so we stay warm and the atmospheric temperature stays stable. Greenhouse gases bounce heat around even to places where there is no direct sunlight, so when you walk into shade, or day turns into night, the temperatures change a little, but not that much. Nowhere on Earth can you stand with half your body in sun and half in the shade and have half your body cooking while the other half is freezing. This could happen on our next door neighbor the Moon, though, because it has no greenhouse gases.
So we have carbon dioxide and other greenhouse gases to thank for us not having to wear spacesuits to protect us from extreme temperatures as we walk around the Earth. These gases keep our atmosphere warm and our temperatures stable. Without them, life as we know it on Earth would not exist.
You may now be wondering, if carbon dioxide keeps us alive, then why are we worried about adding more carbon dioxide to the atmosphere? You can find out the answer to that here (it's another blog post by me).
So far, I don't have any books about carbon dioxide, but if you want to check out some of my books anyways, go here.
Online References and Resources:
Alice Bell. "Climate histories. The oft-ignored story of Eunice Foote."
https://tinyletter.com/climatestories/letters/climate-histories-the-oft-ignored-story-of-eunice-foote
EPA. "Overview of Greenhouse Gases."
https://www.epa.gov/ghgemissions/overview-greenhouse-gases
NPR. "It's All About Carbon, Episodes 1-5." Animated videos.
https://www.youtube.com/watch?v=EvphJO8VKlc&index=4&list=PL1LsUTp-9_dmq24waaoU_3uNTeEnWKYgz
PBS LearningMedia. "Extreme Temperatures on the Moon."
https://ny.pbslearningmedia.org/resource/ess05.sci.ess.eiu.extemp/extreme-temperatures-on-the-moon/#.WR7_LjOZOnc
Also, this book:
An Ocean of Air by Gabrielle Walker
Photos and Images:
Click the photos and images used above to find their sources.
* (This is that footnote I told you about.) Well kind of. The actual blanket around the Earth is gravity. Gravity is what traps the air molecules in our atmosphere, and the air molecules are what hold the heat (just like blankets trap the air molecules heated by your body or a greenhouse traps air warmed by the sun). The analogy breaks down though because gravity isn’t something covering the atmosphere like a blanket. It is a force from the Earth that pulls everything towards it.